|
3-D culture systems

|
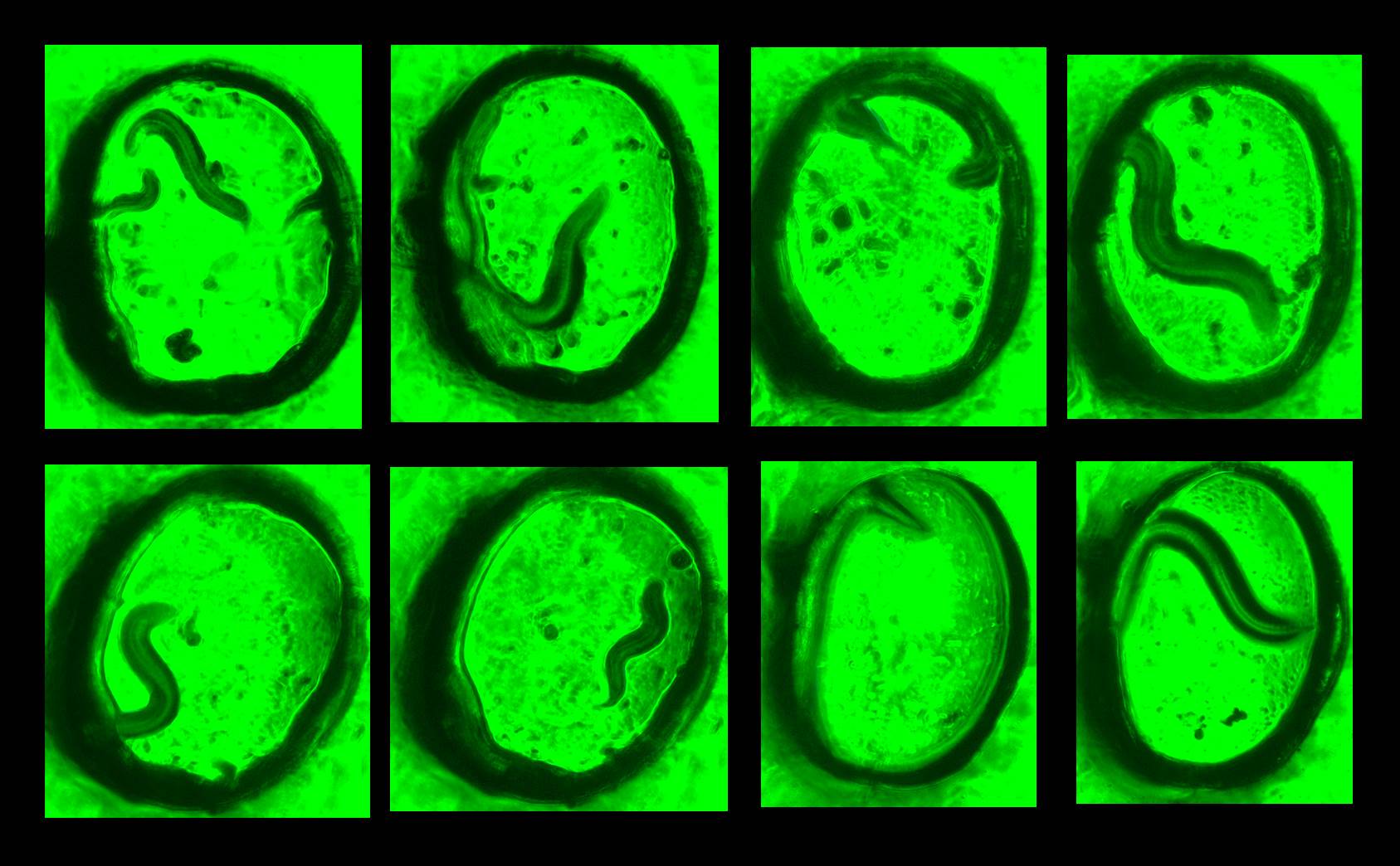
Caenorhabditis elegans is a widely used model organism to study mechanisms of neurobiological processes and human diseases. Developmental transitions in C. elegans larval stages and their reproduction efficiencies rely extensively on cultivating techniques. Typically, C. elegans are cultured using E. coli strain OP50 as a food source on the standard 2-D Nematode Growth Medium (NGM) agar. The cultured worms on the 2-D plate, however, are not an accurate model of their in vivo environment such as rotting fruits and vegetation and there are limitations in understanding their 3-D locomotion behavior at different larval stages during cultivation. Furthermore, we have little control of efficient nutrient/food transport in 3D. Recently, we and other groups developed novel paper-based culture systems for human and other bacterial cells. The paper-based devices provided a new strategy for 3-D cell cultures with controlled mass transport of oxygen and nutrients. By controlling the thickness of the overall biological construct in a multiple-layer paper stack, a 3-D tissue or biofilm model was developed, ultimately increasing our knowledge of its underlying biophysical mechanisms. However, reported work on the 3-D culture of C. elegans is quite limited. Even the latest advances in their 3-D controlled environment do not accurately replicate their natural habitats and do not show any significant difference in culturing compared to the standard 2-D method. In this project, we created a novel paper-based microfluidic platform for 3-D cultivation of the nematode Caenorhabditis elegans. The device utilized multi-laminate structure of transparent polycarbonate papers as a scaffold to mimic the worm¡¯s 3-D natural habitats. Because the paper is thin, mechanically strong and largely porous, the paper-supported structure allowed C. elegans to move and behave freely in three dimensions, and promoted the efficient growth of both C. elegans and their primary food, E. coli bacteria. The transparency of the paper facilitated visualization of the worms under a microscope and sharpened our understanding of their 3-D behavioral dynamics. Development and fertility of C. elegans in the paper-based 3-D device outperformed those of the standard 2-D cultivation technique.
|

